

Employs methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible.All facilities performing any clinical Laboratory testing are required to obtain a CLIA certificate.
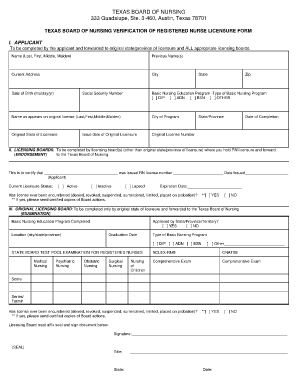
The Centers for Medicare and Medicaid Services (CMS) administers the CLIA Laboratory certification program in conjunction with the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). Blood glucose- devices cleared by the Food and Drug Administration of home useįederal Requirements for a Certificate of Waiver:.Erythrocyte sedimentation rate (ESR) non-automated.



 0 kommentar(er)
0 kommentar(er)
